Abstract
Background
Asparaginase is a key component of pediatric therapy for Acute lymphoblastic leukemia (ALL). Two formulations available in the USA are pegylated E. coli asparaginase (PEG) and Erwinia chrysanthemi asparaginase (Erwinaze). Hypersensitivity reactions have been observed in ~ 20% of patients receiving PEG. Patients with overt symptoms of hypersensitivity are typically switched to Erwinaze. In cases of hypersensitivity, anti-asparaginase antibodies may be present that neutralize circulating asparaginase and in some cases may present without clinical signs, a phenomenon known as silent inactivation. Few studies have prospectively evaluated the incidence of silent inactivation in pediatric ALL patients. In this prospective study, we performed therapeutic drug monitoring of asparaginase levels on newly diagnosed pediatric ALL patients to determine the incidence of silent inactivation and to switch patients to Erwinaze, if silent inactivation was detected.
Methods
A prospective study was conducted at CHOC Children's Hospital on patients with newly diagnosed ALL or lymphoblastic lymphoma between 2016 to 2018. Patients designated as high or very high risk B-ALL post induction, T-ALL and B/T lymphoblastic lymphoma were enrolled. Patients received therapy on or according to a COG protocol with PEG (2500 IU/m2) administered via 2-hour i.v. infusion without antihistamine or steroid premedication. Asparaginase activity was measured days 7 and 14 after each of the two PEG doses during Consolidation. Serum asparaginase level of >0.1 IU/ml and >0.02 IU/ml was considered therapeutic at 7 or 14 days post PEG, respectively. Silent inactivation was defined as serum asparaginase levels below these values without signs of overt allergy. Any patient found to have silent inactivation was immediately switched to Erwinaze. Patient characteristics, common adverse events associated with asparaginase and serum asparaginase levels were analyzed. Descriptive statistics and Pearson's coefficient was used for analysis.
Results
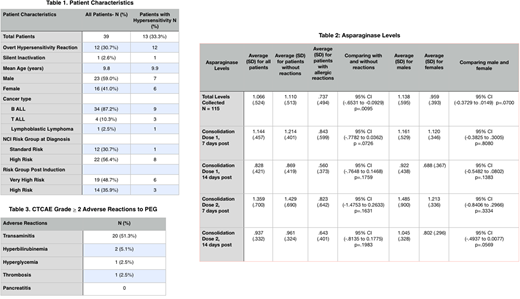
Thirty-nine patients were enrolled with a mean age of 9.8 years (range, 1-23) with the majority being B-ALL (87.2%) of which 3 were Ph-like (Table 1). Twelve patients (30.7%) had overt hypersensitivity and one patient (2.6%) had silent inactivation. One patient had overt hypersensitivity after completion of the PEG infusion with a measured asparaginase level 7 days post that was therapeutic. One-hundred fifteen drug levels were collected, for an average of 2.9 levels per patient (maximum 4 levels). There was a difference in mean asparaginase activity in patients without hypersensitivity compared to those with hypersensitivity (1.110 IU/mL versus 0.737 IU/mL, respectively, p=.0095). There were no significant differences in asparaginase levels based on age, gender or BSA. The highest asparaginase level observed was 7 days post the 2nd dose of asparaginase in Consolidation. Overall, the incidence of patients with CTCAE grade ≥2 transaminitis was 51.3% (n=20), hyperglycemia 2.6 % (n=1), hyperbilirubinemia 5.1% (n=2), and thrombosis 2.6% (n=1). Pancreatitis of any grade was not observed in this cohort.
Discussion
We detected silent inactivation in a single patient who had an asparaginase level of 0.01 seven days after the first dose of PEG and showed no signs of overt allergic reaction. This patient was switched to Erwinaze and an asparaginase level drawn 48 hours post Erwinaze was therapeutic at 0.719. Another patient developed a hypersensitivity reaction 12-hours after PEG and had a serum asparaginase level of 1.29 7-days post PEG. This suggests that not all anti-asparaginase antibodies are neutralizing since this patient continued to demonstrate therapeutic drug levels. The incidence of allergic reactions to PEG at our institution appears higher than reported in prior studies. Our data shows a statistically significant difference between serum asparaginase levels in patients with and without hypersensitivity. Our study also shows that silent inactivation exists in a very small proportion of patients. Additionally, patients who develop late allergic reactions to PEG may still have therapeutic asparaginase levels. Thus, monitoring of asparaginase levels may be warranted to detect silent inactivation and to monitor asparaginase levels in patients who develop delayed hypersensitivity. A larger prospective study is needed to confirm these results.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal